
CGMP
CGMP refers to the Current Good Manufacturing Practice regulations enforced by the FDA (U.S. Food and Drug Administration). CGMPs provide for systems that assure proper design, monitoring, and control of manufacturing processes and facilities. Adherence to the CGMP regulations assures the identity, strength, quality, and purity of drug products by requiring that manufacturers of medications adequately control manufacturing operations.
CGMP stands for Current Good Manufacturing Practice, and it refers to the regulations enforced by the U.S. Food and Drug Administration (FDA). These regulations ensure that various products intended for human consumption and use are produced in a manner that upholds their quality, safety, and efficacy. The “Current” in CGMP emphasizes the need for manufacturers to employ technologies and systems that are up-to-date in order to comply with the regulations. These practices are crucial in the pharmaceutical, biotechnology, medical devices, and food industries, among others.
The main objective of CGMP is to ensure that products are made consistently and that their production meets quality standards. CGMP covers all aspects of production; from the raw materials, the facilities and equipment, to the training and personal hygiene of staff. Proper management of manufacturing processes, accurate documentation, and adherence to prescribed procedures are crucial aspects of CGMP. These measures prevent errors and contamination, thus safeguarding consumer health by ensuring that products do what they are intended to do without causing harm.
CGMP regulations are flexible to a certain extent, allowing companies to decide individually how to best implement the necessary controls. This flexibility permits companies to use modern technologies and innovative approaches to achieve higher quality through continual improvement. Accordingly, the CGMP environment requires rigorous testing of ingredients, materials, and products at critical stages of production to detect any potential risks to quality.
Compliance with CGMP is essential not just for maintaining product quality but also for upholding a company’s reputation and avoiding legal issues or recalls. The FDA inspects manufacturing facilities to check that they comply with CGMPs, ensuring that the products distributed are safe and effective. Non-compliance can lead to serious consequences including recalls, sanctions, and legal action. For consumers, CGMP means increased confidence in the safety and effectiveness of products like medications and food items.
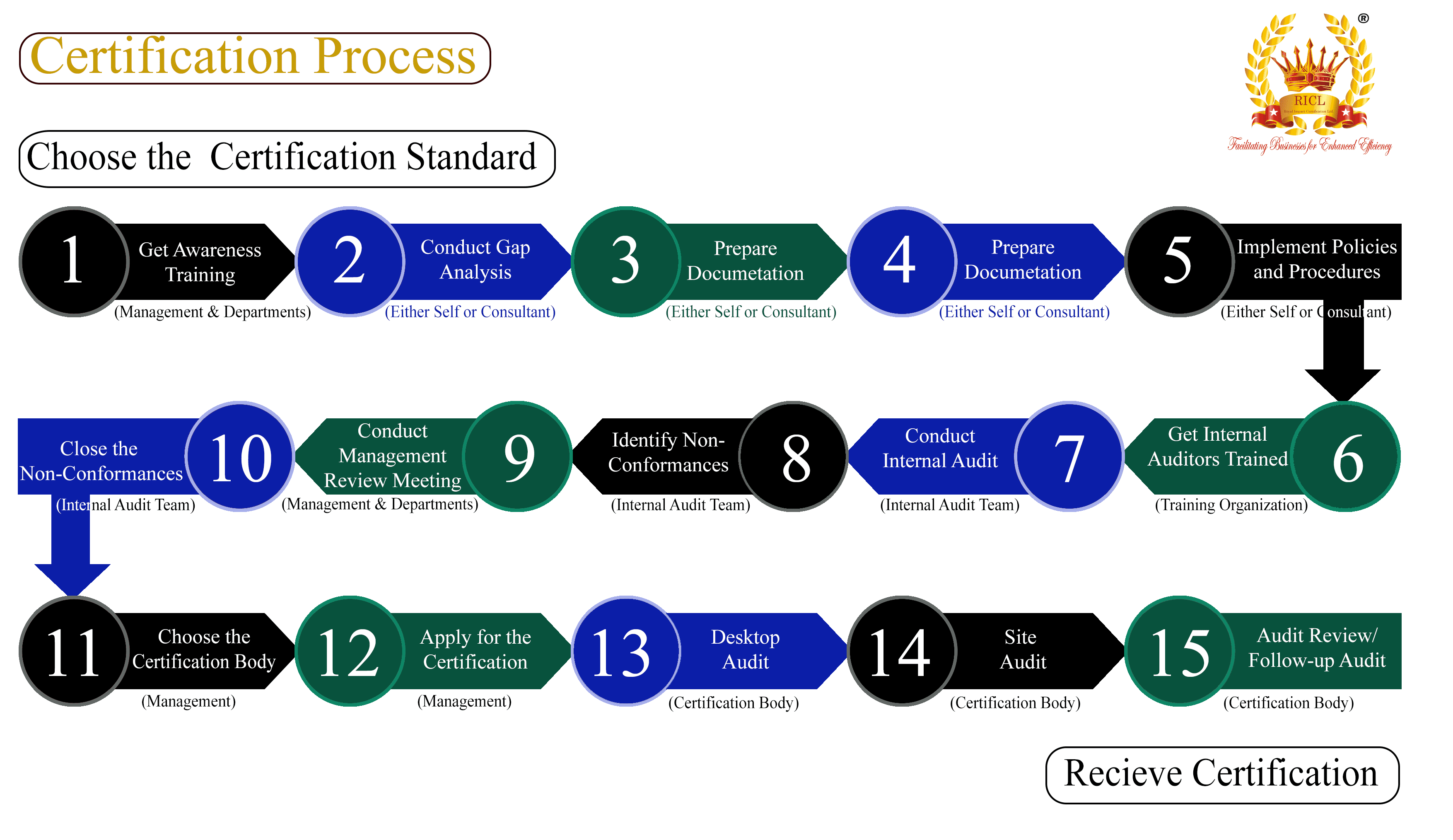
Here are the industries where Current Good Manufacturing Practices (CGMP) are crucial for ensuring quality, safety, and regulatory compliance:
- Automotive manufacturing
- Aerospace industry
- Chemical production
- Electronics manufacturing
- Pharmaceutical industry
- Textile and apparel manufacturing
- Food and beverage production
- Packaging industry
- Printing and publishing
- Construction and building materials
- Oil and gas extraction
- Mining and mineral processing
- Metal fabrication and machining
- Plastics and rubber products
- Renewable energy sector
- Waste management and recycling
- Water treatment and sanitation
- Agriculture and agribusiness
- Forestry and logging
- Pulp and paper industry
- Steel and metal production
- Cement and concrete manufacturing
- Shipbuilding and maritime industry
- Power generation and utilities
- Telecommunications industry
- Transportation and logistics
- Healthcare and medical services
- Education and research institutions
- Hospitality and tourism
- Retail and consumer goods
- Financial services
- Information technology (IT) sector
- Consulting and professional services
- Real estate and property management
- Chemical distribution and trading
- Automotive aftermarket services
- Environmental consulting firms
- Renewable energy developers
- Industrial cleaning and maintenance
- Laboratory and testing services
- Environmental advocacy organizations
- Government and public sector agencies
- Non-profit organizations
- Event management and entertainment
- Sports and recreation facilities
- Textile recycling and upcycling
- Food service and catering
- Green building and architecture
- Eco-tourism and outdoor activities
- Waste-to-energy projects
[sp_easyaccordion id=”10788″]
[formsapp id=”662f63f6c4dd546c9916c3db”]
